How Fuel Cells Work
Polymer Electrolyte Membrane (PEM) fuel cells used in automobiles—also called Proton Exchange Membrane fuel cells—use hydrogen fuel and oxygen from the air to produce electricity. The diagram and animation below show how a PEM fuel cell works.
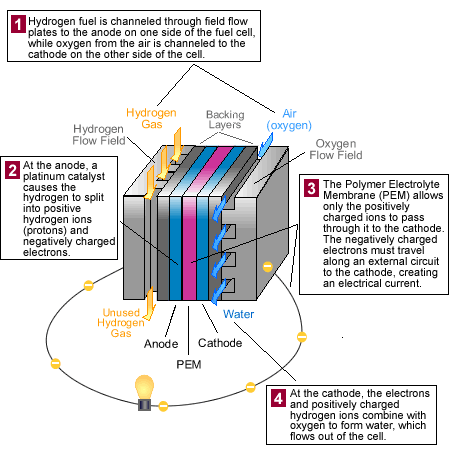
(Flash 5.0 or higher required)
Fuel Cell Stacks
Most fuel cells designed for use in vehicles produce less than 1.16 volts of electricity—far from enough to power a vehicle. Therefore, multiple cells must be assembled into a fuel cell stack. The potential power generated by a fuel cell stack depends on the number and size of the individual fuel cells that comprise the stack and the surface area of the PEM.



